Human Anthrax Toxin Receptor 2 (ANTXR2) ELISA Kit

two product lines: Traditional ELISA Kit and Ready-to-Use ELISA Kit.


Other names:CMG-2; CMG2; ISH; JHF; Capillary Morphogenesis Protein 2
Function: Necessary for cellular interactions with laminin and the extracellular matrix.
Sequence:
50 MVAERSPARS PGSWLFPGLW LLVLSGPGGL LRAQEQPSCR RAFDLYFVLD 100 KSGSVANNWI EIYNFVQQLA ERFVSPEMRL SFIVFSSQAT IILPLTGDRG 150 KISKGLEDLK RVSPVGETYI HEGLKLANEQ IQKAGGLKTS SIIIALTDGK 200 LDGLVPSYAE KEAKISRSLG ASVYCVGVLD FEQAQLERIA DSKEQVFPVK 250 GGFQALKGII NSILAQSCTE ILELQPSSVC VGEEFQIVLS GRGFMLGSRN 300 GSVLCTYTVN ETYTTSVKPV SVQLNSMLCP APILNKAGET LDVSVSFNGG 350 KSVISGSLIV TATECSNGIA AIIVILVLLL LLGIGLMWWF WPLCCKVVIK 400 DPPPPPAPAP KEEEEEPLPT KKWPTVDASY YGGRGVGGIK RMEVRWGDKG 450 STEEGARLEK AKNAVVKIPE ETEEPIRPRP PRPKPTHQPP QTKWYTPIKG 480 RLDALWALLR RQYDRVSLMR PQEGDEVCIW ECIEKELTA
INTENDED USE
The kit is a sandwich enzyme immunoassay for the in vitro quantitative measurement of ANTXR2 in human tissue homogenates or other biological fluids.
DETECTION RANGE
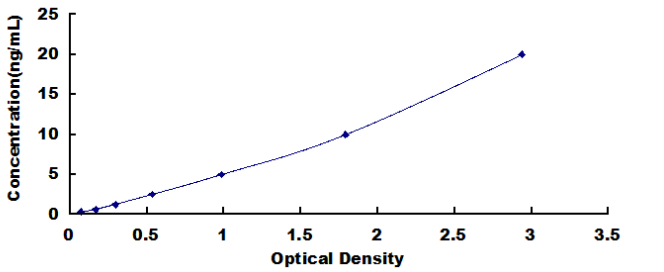
0.312-20ng/mL. The standard curve concentrations used for the ELISA’s were 20ng/mL, 10ng/mL, 5ng/mL, 2.5ng/mL, 1.25ng/mL, 0.625ng/mL, 0.312ng/mL.
SENSITIVITY
The minimum detectable dose of ANTXR2 is typically less than 0.133ng/mL.
The sensitivity of this assay, or Lower Limit of Detection (LLD) was defined as the lowest protein concentration that could be differentiated from zero. It was determined by adding two standard deviations to the mean optical density value of twenty zero standard replicates and calculating the corresponding concentration.
SPECIFICITY
This assay has high sensitivity and excellent specificity for detection of ANTXR2.
No significant cross-reactivity or interference between ANTXR2 and analogues was observed.
You can reference link of the kit as following
https://dldevelop.com/Research-reagent/dl-antxr2-hu.html
https://www.dldevelop.com/uploadfile/data/DL-ANTXR2-Hu.pdf
Introduction
| Item | Standard | Test | |
| Description |
The kit is a sandwich enzyme immunoassay for the in vitro quantitative measurement of ANTXR2 in human tissue homogenates or other biological fluids. |
Conform | |
| Identification | Colorimetric | Positive | |
| Composition | Traditional ELISA Kit | Ready-to-Use ELISA KIT | Conform |
| Pre-coated, ready to use 96-well strip plate 1 | Pre-coated, ready to use 96-well strip plate 1 | ||
| Plate sealer for 96 wells 2 | Plate sealer for 96 wells 2 | ||
| Standard 2 | Standard 2 | ||
| Diluents buffer 1×45mL | Standard Diluent 1×20mL | ||
| Detection Reagent A 1×120μL | Detection Solution A 1×12mL | ||
| Detection Reagent B 1×120μL | Detection Solution B 1×12mL | ||
| TMB Substrate 1×9mL | TMB Substrate 1×9mL | ||
| Stop Solution 1×6mL | Stop Solution 1×6mL | ||
| Wash Buffer (30 × concentrate) 1×20mL | Wash Buffer (30 × concentrate) 1×20mL | ||
| Instruction manual 1 | Instruction manual 1 | ||
Test principle
The microtiter plate provided in this kit has been pre-coated with an antibody specific to the index. Standards or samples are then added to the appropriate microtiter plate wells with a biotin-conjugated antibody preparation specific to the index. Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. After TMB substrate solution is added, only those wells that contain the index, biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450nm ± 10nm. The concentration of the index in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Recovery
Matrices listed below were spiked with certain level of recombinant ANTXR2 and the recovery rates were calculated by comparing the measured value to the expected amount of the index in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 81-93 | 86 |
| EDTA plasma(n=5) | 80-97 | 88 |
| heparin plasma(n=5) | 90-101 | 95 |
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of the index and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 82-96% | 83-98% | 81-99% | 93-101% |
| EDTA plasma(n=5) | 88-101% | 86-95% | 90-102% | 80-93% |
| heparin plasma(n=5) | 80-91% | 82-90% | 95-104% | 79-95% |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level the index were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level the index were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Stability
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage conditions.
Note:
To minimize unnecessary influences on the performance, operation procedures and lab conditions, especially room temperature, air humidity and incubator temperatures should be strictly regulated. It is also strongly suggested that the whole assay is performed by the same experimenter from the beginning to the end.
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 2 hours at 37℃;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37℃;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 1 hour at 37℃;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 15-25 minutes at 37℃;
8. Add 50µL Stop Solution. Read at 450nm immediately.
Order or get a Quote
We will reply you within 24 hours!














